Is FDA investigating Physicians using Illegal Botox?
By Dr. Stephen Cosentino
PRESIDENT OF EMPIRE MEDICAL TRAINING
Is the FDA investigating Medical Spas and Physician offices for using Illegal Botox ?
 Ever wonder why other aesthetic practices offer Botox© procedures for as little as $8.00 - $10.00 per unit? Have you ever received e-mails from internet pharmacies offering 100 unit vials of Botox© for hundreds less than the pricing from Allergan? Well, odds are the products they are promoting is not Botox© Cosmetic as labeled and packaged for the United States as a prescription item. Though the practice by unethical pharmacies and unsuspecting physicians is now the forefront of a new nationwide investigation by the FDA to crack down on the illegal distribution and uses of imported Botox© not marked or labeled to be used in America.
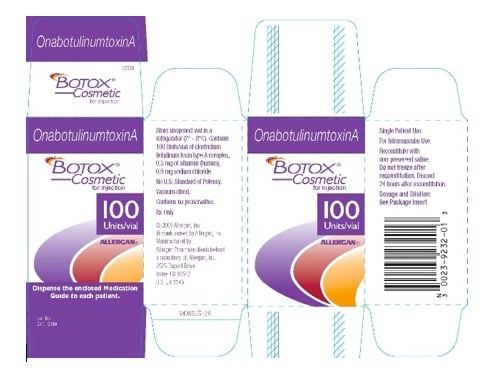
The practice by physicians of using Botox© not marked for distribution or use in the United States has become rampant in recent years as there was little regulatory control to discourage its use. Many physicians considered a gray area since they purchased and received the prescription drug within the United States and many websites exploited this point to persuade doctors that it was OK to purchase and use the drug. However, the FDA has now definitely defined this issue by stating that if the product does not contain the Rx label, labeled for use in the United States, labeled as Botox© Cosmetic, and has the “N” code next to the bar code then it is not approved for use in the United States.
Ever wonder why other aesthetic practices offer Botox© procedures for as little as $8.00 - $10.00 per unit? Have you ever received e-mails from internet pharmacies offering 100 unit vials of Botox© for hundreds less than the pricing from Allergan? Well, odds are the products they are promoting is not Botox© Cosmetic as labeled and packaged for the United States as a prescription item. Though the practice by unethical pharmacies and unsuspecting physicians is now the forefront of a new nationwide investigation by the FDA to crack down on the illegal distribution and uses of imported Botox© not marked or labeled to be used in America.
The practice by physicians of using Botox© not marked for distribution or use in the United States has become rampant in recent years as there was little regulatory control to discourage its use. Many physicians considered a gray area since they purchased and received the prescription drug within the United States and many websites exploited this point to persuade doctors that it was OK to purchase and use the drug. However, the FDA has now definitely defined this issue by stating that if the product does not contain the Rx label, labeled for use in the United States, labeled as Botox© Cosmetic, and has the “N” code next to the bar code then it is not approved for use in the United States.
- Access to the Physician Network of Allergan
- Access to HA Dermal Fillers (i.e. Juvederm Ultra© & Juvederm Ultra Plus©)
- Access to collagen products; Cosmoderm© and Cosmoplast©
- Access to Latisse© and cosmeceutical line; MD Forte©
- Enrollment in Allergan Rewards rebate program
- Marketing support (referral program, brochures, promotions, training materials, etc.)


