Cellulite, or a dimpling effect in the skin which can be a part of the aging process, is estimated to impact 90% of women. But for how widely experienced cellulite is, and how eager patients are to combat its signs, there has until recently been very few options in reducing cellulite with maximum medical efficacy.
In July 2020, Endo International announced that the US Food and Drug Administration had approved Qwo (Collagenase clostridium histolyticum-aaes) for use in treating moderate to severe cellulite in the buttocks of adult women, becoming the first FDA-approved injectable treatment for cellulite. This approval opens up incredible options in a billion-dollar industry and gives practitioners the opportunity to offer their patients the chance for real, effective results without the need for invasive procedures.
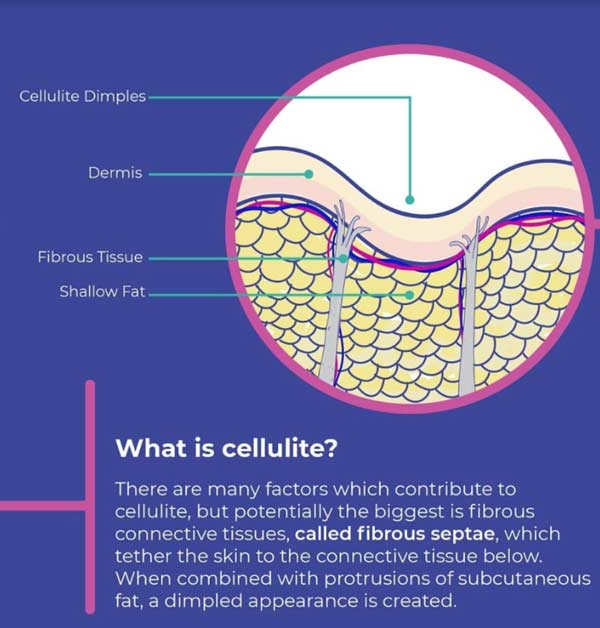
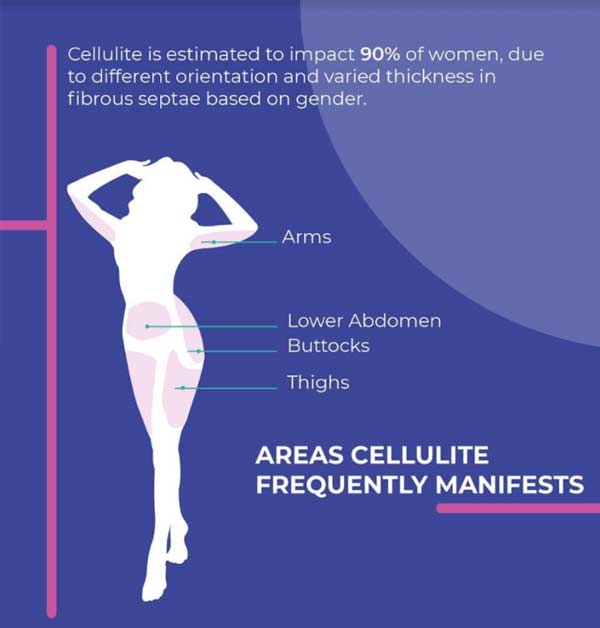
There are many factors which contribute to cellulite, but potentially the biggest is fibrous connective tissues, called fibrous septae, which tether the skin to the connective tissue below. When combined with protrusions of subcutaneous fat, a dimpled appearance is created. Cellulite manifests most frequently on the buttocks, thighs, lower abdomen and arms, and tends to impact women much more frequently than men, due to different orientation and varied thickness in fibrous septae based on gender.
Women also have fewer Beta receptors in areas such as the hips, thighs, and upper arms, areas that are commonly prone to cellulite. Estrogen also inhibits catecholamine-mediated lipolysis in the cells, and these hormone changes over time can cause cellulitic changes in the subcutaneous fat layer. Additional factors can include multiple pregnancies (brings on hormonal changes and venous insufficiency), congenital predispositions, smoking (decreases oxygenation of fat layers), poor diet, impaired venous and lymphatic drainage, and a genetic predisposition to smaller subcutaneous fatty chambers.
There are ways to reduce the appearance of cellulite, many of them lifestyle based: an increased fiber diet, weight loss, exercise, even applying self-tanner to attempt to mask the dimpling effect. But despite women spending an estimated $3 billion a year on anti-cellulite treatments, the vast majority of them are on over-the-country products and not approved by the FDA. But the approval of Qwo injectable treatments by the FDA brings incredible options for treatment, without requiring invasive procedures or surgery.
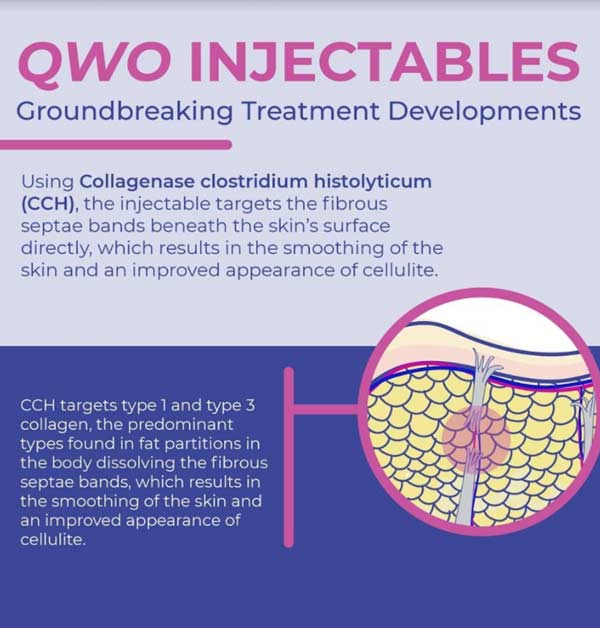
QWO INJECTABLES: GROUNDBREAKING TREATMENT DEVELOPMENTS
Collagenase clostridium histolyticum (CCH) utilizes collagenase, an enzyme that helps break down collagen, and in the case of CCH specifically targets type 1 and type 3 collagen, the predominant types found in fat partitions in the body. The injectable targets the fibrous septae bands beneath the skin’s surface directly, which results in the smoothing of the skin and an improved appearance of cellulite.
Qwo’s CCH treatment consists of up to three treatment sessions, spaced around 21 days apart. In clinical trials of over 800 recipients, the goal was to see a two-grade improvement of the severity of their cellulite. Improvement was seen three weeks after the injection, and continued to show even better results as time went on. For some patients with more severe cellulite, additional treatments could be required.

Qwo was developed by Endo International and currently is priced from $1,800 to $3,200, depending on the size of the affected area and the number of sessions needed. Qwo has been made available to the public as of Spring 2021.
Empire Medical Training offers a great course in Mesotherapy which explains how to reduce the appearance of cellulite and work towards removing it forever.